 DIY
Glycolysis
DIY
Glycolysis DIY
Glycolysis
DIY
GlycolysisBack to Strategy, forward to Keeping Track.
In the transformation of glucose to lactate the hexose is split into two halves and the atoms are rearranged. The empirical formulae of glucose and lactic acid are;
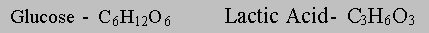
So it's possible to make two moles of lactic acid from one mole of glucose with no net addition or removal of atoms.
Although lactic acid has the right empirical formula for a monosacharide carbon 1 is more oxidised and carbon 3 is less oxidised than the closest monosacharide - glyceraldehyde. So you need to split glucose into two three carbon monosacharides and then rearrange.
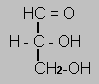 Glyceraldehyde
Glyceraldehyde 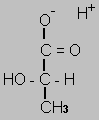 Lactate
Lactate
Author: Jon Maber
j.r.maber@leeds.ac.uk
Dept Biochemistry & Molecular Biology, The University of Leeds, U.K.