 Introduction
to Glycolysis
Introduction
to Glycolysis Introduction
to Glycolysis
Introduction
to Glycolysis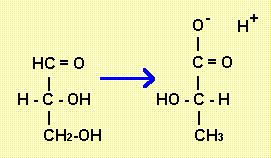
In anaerobic conditions there is no overall oxidation of the monosacharide so why is there a free energy change big enough to be used to make ATP? The answer comes from comparing the structure of glyceraldehyde with the structure of lactate. The conversion of glyceraldehyde to lactate is the net result of the last two stages of glycolysis. This conversion involves a simple swap of atoms. (Although achieved in several individual reactions.)
Video for Windows Animation 18k
As you know, oxidation is loss of electrons. In aerobic respiration it is molecular oxygen which ultimatly accepts electrons from 'fuel' molecules. Although there is no loss of electrons in anaerobic glycolysis, there is a certain amount of partial transfer of electrons from the carbon and hydrogen atoms to the oxygen atoms within the fuel molecule. In essence, some atoms partly 'oxidise' others and are themselves 'reduced'.
When the atoms of glyceraldeyhde are rearranged to form lactate a -COOH group is formed. The bonding electrons in this group are more mobile than in most bonds of organic molecules and so the electronegetive oxygen atoms are able to pull them towards their nuclei. In a way there is an internal, partial redox. This is also the reason why the group is acidic - the electrons in the O-H bond are pulled away from the hydrogen nucleus so hard that the bond breaks.
When electrons move closer to atoms which attract them there is a drop in potential energy and so there is energy available to do work. i.e. make ATP from ADP and phosphate. It's possible that stages two and three evolved earlier than stage one in anaerobic organisms which utilised sugars simpler than glucose.
Author: Jon Maber
j.r.maber@leeds.ac.uk
Dept Biochemistry & Molecular Biology, The University of Leeds, U.K.